westernman
Well-Known Member
And have more than one to improve the chances.I don't think there is any perfect position ... too many factors like airflow etc. can affect it ... all we can hope is that we get it as best as possible.
And have more than one to improve the chances.I don't think there is any perfect position ... too many factors like airflow etc. can affect it ... all we can hope is that we get it as best as possible.
And air is mostly N2 (about 78%), which has the same molecular weight as CO! As far as I can see, the main thing is that CO comes from flames, so it will be hot and rise at least initially. As you say, diffusion will be a relatively slow process.The arguments and statements about 'Carbon Dioxide is heavier than air so will move down', which also is said about propane or butane gas, are all complete boll*^£s. There's a difference between a gas and a liquid; in a gas there is no displacement, the molecules are not bonded together and all bounce around in thermal equilibrium and just mix since the distance between molecules is vast compared to the distance over which they interact, ie they just bounce off each other like billiard balls. It's what gas means!
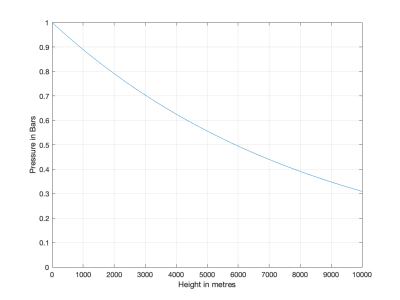
To calculate the change with height you have to apply the Boltzmann distribution, which is that at a height x the number of molecules per volume,
n(x) = n(0) exp(-E(x)/kT)
where k is the Boltzmann constant, T the temperature in Kelvin and E(x) is the energy of a single molecule as a function of height. E = mgx, where m is the mass of the molecule, g the acceleration due to gravity (9.81ms-2 and x the height. The mass of a molecule of Nitrogen (air is 4/5 Nitrogen) is 28 / NA grammes where NA is Avogadro's number, thus 0.028 / 6x1023 kg.
Putting these into the formula (and assuming temperature doesn't vary much) one gets the reduction in the number of molecules with height as shown here:
View attachment 183993
which shows the expected reduction to 0.7 atmospheres at a height of 3000 m.
More to the point, the difference in concentrations of CO and O2 over 2 metres height will only be 3.3x10-5, or one part in 30,000. Gasses just don't collect in the bilge or rise up - they just disperse in all directions, very fast if small molecules, rather slower for very large molecules.