merjan
Member
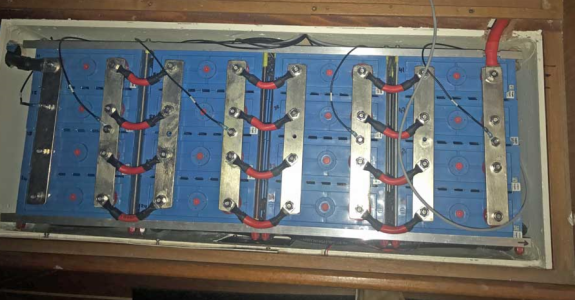
I'm wondering if tinned copper busbars are literally what it says on the tin(!) or are there special processes / materials invovled. I'm thinking of making tinnned copped busbars at home with electrolysis, mostly because it would be fun and less to save money. If I achieve a nice tin coat on a copper plate, would it be expected to conduct as well as an industrially manufactured busbar?
Last edited: