Poignard
Well-Known Member
Yes but I still wanted it turned off at the bottle.Didn't you have an on/off valve near the cooker?
Yes but I still wanted it turned off at the bottle.Didn't you have an on/off valve near the cooker?
Another origo convert. Much safer too. A little slower but who's in a hurry.
Yes but I still wanted it turned off at the bottle.
Utter bollux.LPG gas is not lighter than air.
LPG (propane) is heavier than air.
Butane is also heavier than air.
In fact, LPG is over 50% heavier than air at sea level (1 atm).
As a result, LPG gas will settle in low places
....
...
Do you have gas at home? If so, do you turn it off at the mains when you're not using it?
Utter bollux.
It's a gas.
It will obey the gas laws.
I suggest you lie on the floor so that some of the oxygen that will have settled there gets to your brain.
Or not.
LPG gas is not lighter than air.
LPG (propane) is heavier than air.
Butane is also heavier than air.
In fact, LPG is over 50% heavier than air at sea level (1 atm).
As a result, LPG gas will settle in low places
Thus, using Butane (C4H10 -> 58 g / mole), and a locker height of 50cm gives the variation of concentration of Butane over the locker of 0.01%: put another way, at the top of the locker it's 99.99% as concentrated as it is at the bottom of the locker.
Utter bollux.
It's a gas.
It will obey the gas laws.
I suggest you lie on the floor so that some of the oxygen that will have settled there gets to your brain.
Or not.
A mundane reason for having a drain at the base of any compartment, which falls to the overboard discharge point, is so that any common or garden water that gets in can take itself out again.
I got an Origo two burner and ditched the gas system on my boat. Mind you, it's a small boat at 31 feet and we never used the oven. It's efficient, wont explode and fire can be doused with water. I'm burning clean Bio Ethanol so other advantages are no smell and no Carbon Monoxide.
Gas is heavier than air and if it gets into your bilges its hard to shift without turning the boat upside down.
you can have a pinhole leak in your inboard hose and you will know nothing until BOOM!
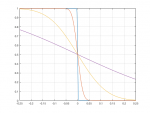
i find it amazing that there are so many experts on these forums when the info came from a gas producer on the internet, gas can only mix and move around if there is air flow to mix the gases
That is simply not true. Diffusion will mix gases over time even with no large scale fluid motion. The only exceptions are hydrogen and helium which are so much less dense than other gases that barometric effects (ie gravity) prevails over diffusion. That's why there is effective no hydrogen or helium in the atmosphere; they both rise over time to the top and then diffuse away into space.
or you can put a vent at the bottom of the locker and let the gas drain out
Any one that does not have a gas vent is a walking time bomb
, already explained that Butane and Air cause a mixture of dangerous explosives even after diffusion in your lovely sealed gas locker you open it and some on lights a match or a candle near by BOOM